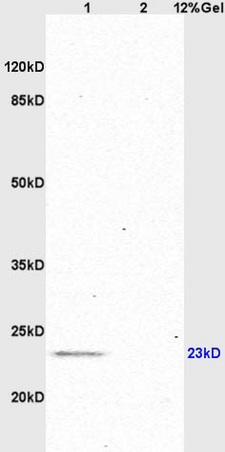
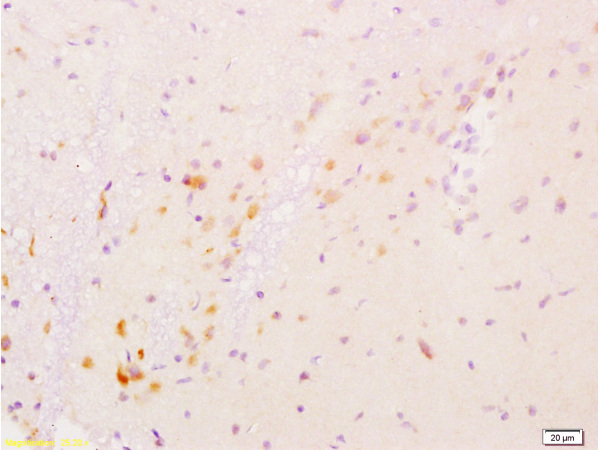
Protein Kinase c alpha (PKC alpha) is an 77 kDa member of the conventional group (cPKCs: sensitive to calcium, diacylglycerol, phosphatidylserine and phorbol esters) of the PKC family of serine/ threonine kinases that are involved in a wide range of physiological processes including mitogenesis, cell survival and transcriptional regulation. PKC alpha is an ubiquitously expressed PKC isozyme that has been implicated in the regulation of a broad range of cellular functions including proliferation, differentiation, development, migration, cell cell adhesion, cell extracellular matrix adhesion, and solute transport. The activation loop threonine (threonine 497 in PKC alpha) of conventional PKCs is phosphorylated by phosphoinositide dependent kinase 1 (PDK1). This phosphorylation is necessary for the autophosphorylation of threonine 638 in the carboxy terminus of PKC alpha, a step that is critical for regulating the rate of PKC alpha dephosphorylation and inactivation.